Europe’s medical device certification ‘CE’ is an international safety certification being available in 21 member states of EU such as Germany and UK and 7 associate members of EU. To sell and manufacture the medical device in Europe, this Certification should be obtained, and only CE products can be sold.
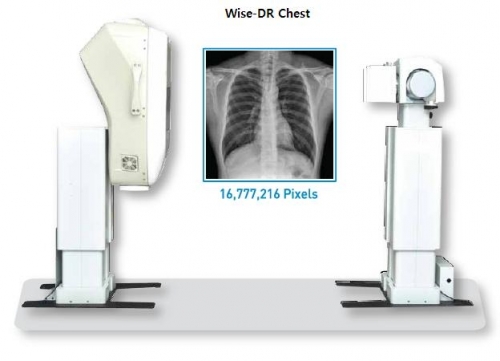
Wise-DR product is a high-function medical device which can acquire an image through a single CCD sensor, as the 3rd generation digital X-ray with 16 mega-pixels of high resolution.
Wise-DR Product has an excellent ergonomic design and can be easily installed and used based on TCP/IP Ethernet. It can not only save a hospital’s working hours through a quick image processing speed but also improve the treatment’s efficiency.
Also, Wise-DR Product provides the high quality medical service through a strong Viewer system. Compared to existing products, its soft grid has been improved and the noise has been markedly reduced by patented sensitivity improvement technology. As it combines the medical image processing software called ‘WiseVue’ and PACS Software, it can provide all of solutions required for the latest digital hospital.
Lee Ju-Sang, CEO of PANANOMICS said, “We are stepping forward to the world market as well as the domestic market by obtaining this CE Certification. We are set to expand the technology area to the nuclear radiation detection field over the X-ray detection field.”
Besides, PANANOMICS has provided about 120 products to hospitals and clinics in Korea in 3 years after entering the digital X-ray market, and has grown to Korea-top company in the 16 mega-pixels equipment. Currently, PANANOMICS has acquired ISO 13485, ISO 9001, GMP Certification in addition to CE Certification, and was awarded a grand prize with ‘Medical X-Ray Detector and Imaging Solution’ at the 3rd national medical device startup competition.
저작권자 © Korea IT Times 무단전재 및 재배포 금지


